by Gary Ciment, Ph.D., Scientific Director, Aves Labs, Inc.
What Are Pannexins?
The pannexins are a gene family related in structure (i.e.,4-pass transmembrane proteins) and function to their more famous cousins -- the gap junction proteins, the connexins (Panchin, 2000). All of these gene family members can form membrane channels that open in response to various intracellular events (e.g., depolarization), and allow for the passage of ions and other small molecules, including ATP. One major difference between the connexins and the pannexins, however, is that pannexin proteins on the surface of adjacent cells don't form functional intracellular connections (i.e., don't form a tunnel linking the cells electrically, like the gap junction proteins do). Instead, the pannexins allow passage of ions and small molecules directly between the intracellular compartment of one cell and the extracellular space around the surrounding cells (Hanstein et al., 2013). In this sense, the pannexins allow for the paracrine release of intercellular signaling molecules, including ATP.
There are three gene products in the pannexin gene subfamily. Pannexin-1 (UniProt Q96RD7, "Panx1") is the most widely expressed. In the peripheral nervous system, Panx1 is found on the surface of sensory neurons and glial cells, including trigeminal ganglionic sensory neurons subserving sensations from the face, including pain sensations (Hanstein et al., 2013) (see also Figure 2, below).
Exploring Possibilities
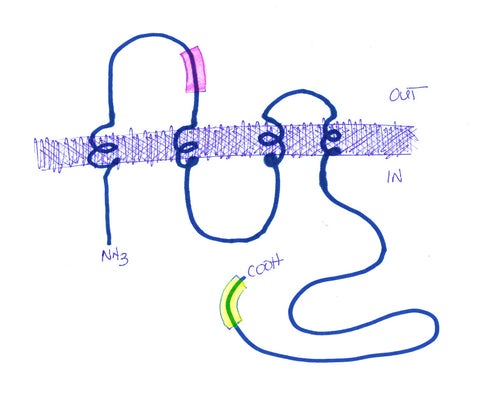
 Dr. Eliana Scemes (Albert Einstein College of Medicine, NY) approached Aves Labs several years ago about making a Panx1-specific antibody (there are also Pannexins -2 and -3, which she did not want the antibody to recognize) that her lab could use for immunohistochemistry and other applications. She was interested in exploring the possibility that the paracrine functions of panx1 may underlie some important aspects of pain perception, and might ultimately serve as useful targets for production of novel analgesic drugs. Using our proprietary Immunogenicity Algorithm®, we identified 2 peptide sequences within the panx1 sequence that fit our criteria for immunogenicity (see Figure 1). From this list, Dr. Scemes chose a sequence near the N-terminus and within the first extracellular loop of panx1. After consultation with Dr. Scemes, we added a cysteine and a synthetic spacer amino acid to the N-terminus of this sequence, and synthesized the 13-mer peptide.
Dr. Eliana Scemes (Albert Einstein College of Medicine, NY) approached Aves Labs several years ago about making a Panx1-specific antibody (there are also Pannexins -2 and -3, which she did not want the antibody to recognize) that her lab could use for immunohistochemistry and other applications. She was interested in exploring the possibility that the paracrine functions of panx1 may underlie some important aspects of pain perception, and might ultimately serve as useful targets for production of novel analgesic drugs. Using our proprietary Immunogenicity Algorithm®, we identified 2 peptide sequences within the panx1 sequence that fit our criteria for immunogenicity (see Figure 1). From this list, Dr. Scemes chose a sequence near the N-terminus and within the first extracellular loop of panx1. After consultation with Dr. Scemes, we added a cysteine and a synthetic spacer amino acid to the N-terminus of this sequence, and synthesized the 13-mer peptide.
The Procedure
Following our standard injection protocol, a KLH-conjugate of this peptide was injected over a 7-week period into the breast muscles of two laying hens, and then eggs were collected over a 3-week period after the 4th injection. From 12 of these eggs, we purified 1500 mg of IgY using our proprietary methods, and from this IgY preparation, we further purified 8.0 mg of affinity-purified antibody. Since the hens were unharmed during the egg collection phase, of course, we had the option of doing additional injections and purifying additional affinity-purified antibody.
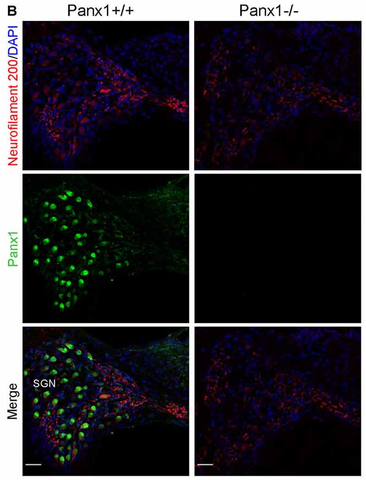
Dr. Schemes and her collaborators then did a set of studies with this antibody looking at the phenomenon of allodynia (a form of pain hypersensitivity, where even innocuous tactile stimuli can produce discomfort) (Hanstein et al., 2016). These studies were performed in wild type mice, panx1-null mice (i.e., no panx1 expression), panx1-glial hypomorphic mice (i.e., panx1 is expressed in non-neuronal cells at abnormally lower levels), and panx1-neuronal hypomorphic mice (i.e., panx1 is expressed in neuronal cells at similar abnormally lower levels).
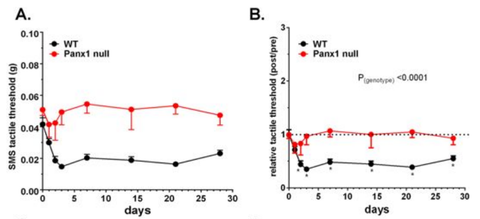
Figure 3 (see below) shows that panx1 plays an important role in the phenomenon of allodynia. In wild type-mice, allodynia could be seen following injection of an irritant into the submandibular skin of the face, as you would fully expect it to. These mice withdrew their faces away from a gentle tactile stimulation of their chin. However, in panx1-null mice, the same extent of allodynia was not seen under the same circumstances.
The Findings
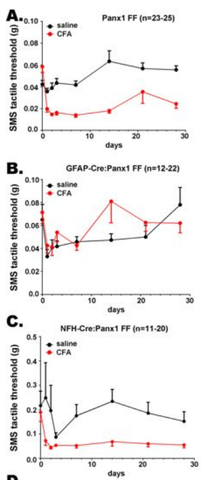
Working with David Spray's laboratory, they noted a very interesting finding in the glial- versus neuronal-hypomorphic mice that they had generated earlier (Hanstein et a., 2013; see also Figure 4). In the neuronal hypomorphic mice, there was only a transient loss of allodynia, but in the glial hypomorphic mice, the failure to see allodynia was long-lasting. This suggests that the non-neuronal cells within sensory ganglia, such as the trigeminal ganglion, are not simply "support cells," but play an active role in sensory information processing, at least in this model of facial pain.
One model that could explain these observations is that transient neuronal activity in pain neurons of the trigeminal ganglion causes local increases in extracellular potassium ions, which depolarize non-neuronal cells within the ganglion. Since depolarization is known to activate panx1, it is reasonable to imagine that the non-neuronal cells then release ATP through the panx1 channel, which acts via purinergic receptors on nearby sensory neurons to alter their sensitivity to tactile stimuli. This model is supported by findings that show that panx1 gene expression is induced in sensory neurons and glial cells following injection of irritants into the target skin of the neurons (Zhang et al., 2015; Hanstein et al., 2016).
This notion would support a radically different and exciting view of sensory glia from passive support cells to ones directly involved in aspects of information processing. If confirmed, these studies would open a whole new set of potential analgesic agents that could potentially avoid the cardiovascular risks associated with non-steroidal anti-inflammatory drugs (e.g., aspirin and ibuprofen) and avoid the addictive properties of opioids.
References
- Hanstein, R., Negoro, H., Patel, N.K., Charollais, A., Meda, P., Spray, D.C., Suadicani, S.O., Scemes, E. (2013). Promises and pitfalls of a Pannexin1 transgenic mouse line. Front. Pharmacol. 4: Article 61, pp. 1-10.
- Hanstein, R., Hanani M., Scemes, E., Spray, D.C. (2016). Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Scientific Reports 6:38266, doi; 10.1038/srep38266.
- Panchin, Y., Kelmanson, I., Matz, M., Lukyanov, K., Usman, N., Lykyanov, S. (2000). A ubiquitous family of putative gap junction molecules. Curr. Biol. 10: R473-474.
- Zhang, Y., Laumet, G., Chen, S. R., Hittelman, W. N., Pan, H. L. (2015). Pannexin-1 Up-regulation in the Dorsal Root Ganglion Contributes to Neuropathic Pain Development. J Biol Chem 290: 14647–14655.
- Zorzi, V., Paciell, F., Ziraldo, G., Peres, C., Mazzarda, F., Nardin, C., Pasquini, M., Chiani, F., Raspa, M., Scavizzi, F., Carrer, A., Crispino, G., Ciubotaru, C.D., Monyer, H., Fetoni, A.R., Salvatore, A.M. , Mammano, F. (2017). Mouse Panx1 Is Dispensable for Hearing Acquisition and Auditory Function Front. Mol. Neurosci. 10: Article 379, pp. 1-17.

